MDR Compliance is Both Hard Work and Costly
The Medical Devices Regulation (MDR) makes launching new products in the market significant more costly and time-consuming, directly impacting your time to market. We believe that all players in the market will be affected and the winners will be the ones that prioritize their efforts best in terms of their existing and future product portfolio.
MDR Disrupts the Industry
Normally when we talk about disruption, we think of innovative disruption caused by a new technology or service. However, disruption can also come from other sources. In the case of MDR, we can talk about legislative disruption.
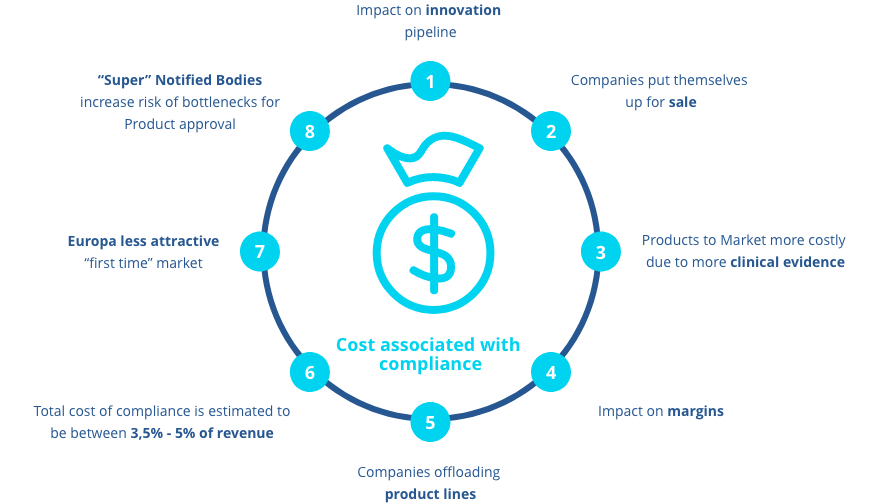
There is a lot of work and a lot of cost associated with becoming MDR compliant. According to a survey by Ernest & Young, the total cost of compliance is estimated to between 3.5% and 5% of revenue. This will affect margins and thereby the financial health of many businesses, forcing them to offload product lines or even put themselves up for sale. Not only will launching new products in the market be significantly more costly due to the demand for more clinical evidence. It will also be more difficult. One bottleneck is the Regulatory Notified Bodies that slows down product approvals. This makes Europe look much less attractive as a “first time” market.
All Players will be Affected
MDR hits small companies hard due to their limited resources and capital. At the same time, it makes larger companies more vulnerable to innovative disruption by forcing them to concentrate their efforts on existing customers and technology. In general, bringing new, innovative products to market will be a challenge for all companies. This means that everyone is forced to prioritize their efforts carefully.
Strategies for Tackling MDR
When it comes to MDR, the winners will be the ones that prioritize their efforts best. To do this, companies must analyze their existing and future product portfolio, grouping products into three categories:
- Today’s products – existing products that are core to the business
- Tomorrow’s products – products for the digital workflow, already known in the market but not currently in portfolio
- Future products – next generation products, unknown to the market
Are your core products MDR compliant?
The first priority is to ensure that all core products are MDR compliant. However, it makes sense to evaluate if any new products should be giving main focus. Focusing on tomorrow's products will put you on pair with the competition after MDR. However, if you utilize partnerships with other companies to ensure that your entire digital product suite is complete, you can focus your company’s resources on next generation products. This gives you a competitive advantage and safeguards your business on the long term.

Use MDR-Ready Components
We offer a suite of MDR-ready components in the digital workflow and can help you complete your offering of tomorrow’s products. This will enable you to focus your efforts on next generation products.
Contact us for an assessment of your MDR readiness.

