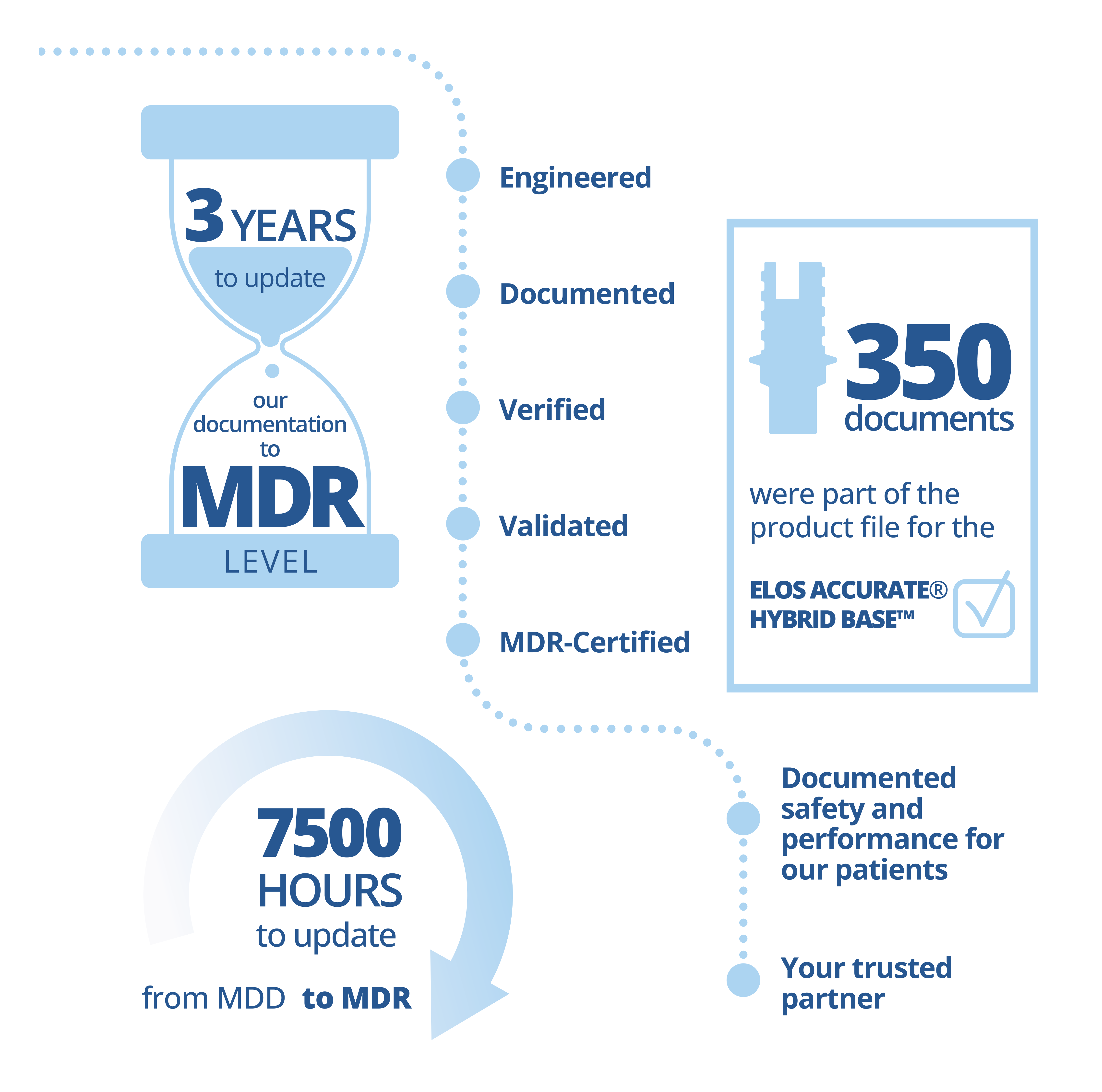
We are excited to share that we have recently received certification for all our Class IIb products in line with the EU Medical Device Regulation (MDR). We are committed to continuously improve and innovate our processes to provide better products and solutions to our customers. As competence is the key to moving forward, all of our Elos Accurate® products are fully certified, ensuring the continuation of reliable supply of products, before and after May 2024, in compliance with relevant standards and regulations.
“We are pleased to receive the MDR certification, an EU regulation to increase the safety and performance of medical devices for patients,” says Henrik Andersen, Head of Engineering of Elos Medtech, Dental.
“As a trusted dental partner, we are in compliance with the standards and regulations that we follow throughout the entire development and manufacturing processes. The accreditation shows our commitment towards excellence, and I would like to thank all my colleagues at Elos Medtech for this incredible achievement.”