New Product catalog 2024
Explore a world of choices: Now More Products to Choose From

The product enables the process
How the new Elos Accurate® Pre-Milled Blank Solutions enable smoother workflows
Elos Accurate® Libraries:
Precision Meets Simplified Efficiency

From initial idea to groundbreaking solution:
The developmental journey of the new Elos Accurate® Pre-Milled Blank Solutions

Elos Medtech Partners with Planmeca
to Provide Customized Titanium Abutments for Planmeca PlanMill 60® S Milling Unit.

Elos Medtech launches Elos Accurate® Pre-Milled Blank Solutions in US:
enables FDA-compliant, in-house milling of custom abutments
How to overcome the challenges involved
when achieving the benefits of in-house milling

Simplifying the journey from initial scan to final smile
Workflow simplification
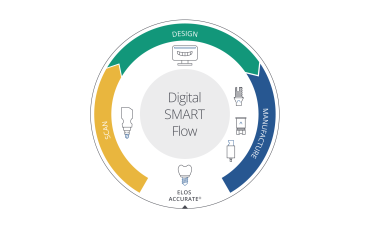
Digital SMART Flow - a smart way into seamless digital dentistry
We know. You must be thinking this is a trick! But it is not.
